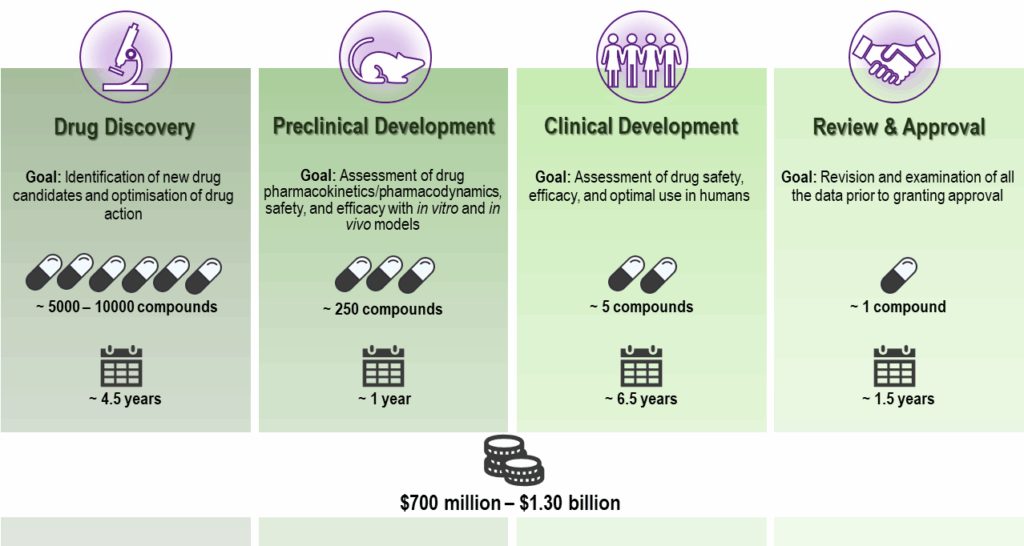
Before any treatment becomes available to patients, it must be proven to be both effective and safe. This involves a long (often 10-15 years), complex and multi-stage process of drug discovery and development. This process can be grouped into 5 stages: Drug Discovery, Preclinical Development, Clinical Development, Regulatory Review and Approval, and Pharmacovigilance. As scientific knowledge evolves, or in certain emergency situations, such as a pandemic, the length of this process can be significantly reduced due to a variety of factors. Nevertheless, all steps and safety checkpoints must always be met before (and after) a new medicine is launched for mass use. The majority of drugs that enter this journey fail at one of these checkpoints – 90% at the last two stages -, thus resulting in significant high costs until a drug candidate succeeds and can be commercialized.
![]() Drug Discovery
Drug Discovery
To find a new potential drug, the process begins with the screening of thousands to millions of compounds. Here, there are two main approaches which can be followed. The screening might aim to find which compounds interact with a known target (e.g., a protein or gene) that is critical in the underlying mechanisms of the disease of interest, or which compounds produce a desired effect (e.g., reducing the multiplication of pathogens such as bacteria or viruses) without first knowing the target or its role in the disease development.
Regardless of the approach, the compounds that show positive results in the screening (called “hits”) are then optimized into “leads” by undergoing improvement of important pharmacological properties, such as selectivity, potency, stability, bioavailability, and solubility.
![]() Preclinical Development
Preclinical Development
This stage comprises laboratory testing of the lead compounds to assess their safety, efficacy, toxicity, and pharmacokinetics (i.e., the movement of the drug into, through, and out of the body – absorption, distribution, metabolism, and excretion). For this, experiments of increasing complexity are conducted, from in vitro (e.g., test tubes and cell assays) to in vivo (animal models).
Out of this vast array of studies, the compounds with the best pharmacological profiles are selected and all the data collected is compiled for regulatory submission. Hence, only a small fraction (about 2%) of the tested compounds moves forward in the drug development process. Importantly, advances in technology, automation, and data analysis are helping to streamline and improve the efficiency of this process.
![]() Clinical Development
Clinical Development
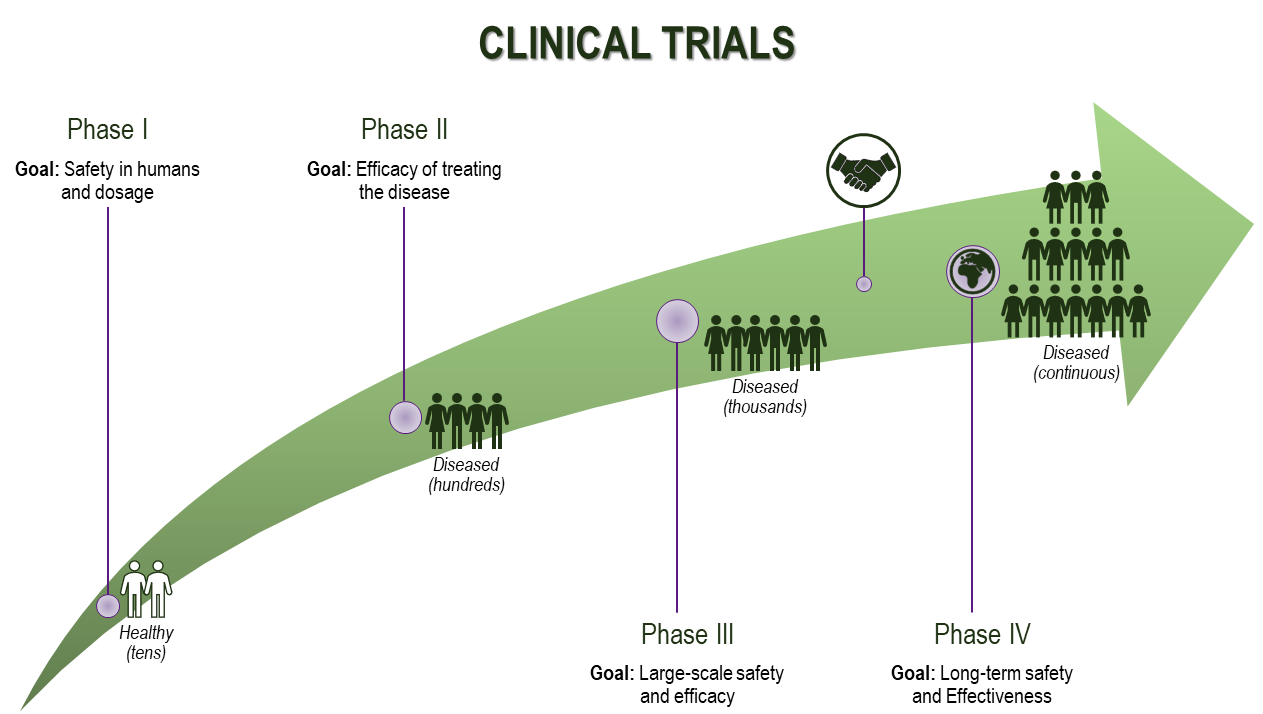
Upon approval, studies in humans (clinical trials) begin in order to collect further data on safety and efficacy as well as optimal dosing and interactions of the test compound under well-controlled monitoring conditions. These studies typically follow three phases:
- Phase I: Small groups (in the order of tens) of healthy volunteers to evaluate safety (no major reactions to the test drug) and dosage.
- Phase II: Larger groups (in the order of hundreds) of patients to assess efficacy and side effects.
- Phase III: Large-scale assessment (usually among different countries) to confirm efficacy, compare with standard treatments, and monitor adverse reactions.
There are exceptions to this traditional phased design, such as clinical trials for rare and life-threatening diseases or in biosimilarity studies (which aim to demonstrate similarity between a novel compound and an existing biologic). Deviations from the typical Clinical Development pathway may include skipping certain phases or running phases in parallel, among others.
![]() Regulatory Review & Approval
Regulatory Review & Approval
When the trials conducted prove successful, a New Drug Application or equivalent is submitted to the appropriate regulatory agencies, e.g., the Food and Drug Administration (FDA) in the United States and the European Medicines Agency (EMA) in Europe. The data collected throughout all stages are reviewed and evaluated not only for safety and efficacy, but also manufacturing quality before marketing approval for the new drug is granted.
![]()
Pharmacovigilance (Post-Marketing Surveillance)
The long process of drug discovery and development does not come to an end with marketing approval. Instead, drugs are continually monitored for long-term safety and effectiveness in the general population (also called Phase IV trials). This stage is extremely important because certain findings regarding adverse reactions and the drug’s real-world performance can only be obtained through larger and more diverse study populations in real-world clinical practice, as this setting is less controlled and standardised than those in the stages prior to approval.
In such a complex and multi-level process, high levels of multidisciplinarity among academic institutions, pharmaceutical companies, technology providers and regulatory authorities are critical to the successful development of novel drugs. To this end, the European consortium ‘Stop Spread Bad Bugs’, funded by the Marie Skłodowska-Curie Actions (MSCA), has been established. Through a doctoral network where academia and industry work in close collaboration, this project focuses on the development and preclinical testing of novel drug candidates to address the alarming public health threat of bacterial multidrug resistance (MDR).
This blog was written by Ariana Carvalho, one of the PhD candidates working in the SSBB consortium. Ariana is pursuing her PhD trajectory at the University of Marburg in Germany and TissueGnostics in Austria.
List of References
- Exploring the five phases of drug development – Patheon pharma services (2023). https://www.patheon.com/us/en/insights-resources/blog/drug-development-phases.html (Accessed 15 April 2025)
- Drug Discovery and Development Process | PPD. https://www.ppd.com/what-is-a-cro/drug-discovery-and-development/ (Accessed 15 April 2025)
- From lab to patient – The journey of a medicine assessed by EMA | European Medicines Agency (EMA) (2019). https://www.ema.europa.eu/en/from-lab-to-patient-timeline (Accessed 15 April 2025)
- The Drug Development Process | FDA (2018). https://www.fda.gov/patients/learn-about-drug-and-device-approvals/drug-development-process (Accessed 15 April 2025)
- Discovery & Development – MCC (2024). https://www.msd.at/en/discovery-development/ (Accessed 17 April 2025)
- Singh, N. et al. Drug discovery and development: introduction to the general public and patient groups. Frontiers in Drug Discovery 3, 1201419 (2023). https://www.frontiersin.org/journals/drug-discovery/articles/10.3389/fddsv.2023.1201419/full
- Lo, B., Field, M. J. & Institute of Medicine (US) Committee on Conflict of Interest in Medical Research, E. and P. The Pathway from Idea to Regulatory Approval: Examples for Drug Development. (2009). https://www.ncbi.nlm.nih.gov/books/NBK22930/
- Drug discovery and development process | Novartis (2011). https://www.youtube.com/watch?v=3Gl0gAcW8rw (Accessed 21 April 2025)
- Van Norman, G. A. Drugs, Devices, and the FDA: Part 1: An Overview of Approval Processes for Drugs. JACC Basic Transl Sci 1, 170–179 (2016). https://pmc.ncbi.nlm.nih.gov/articles/PMC6113160/
- Brown, D. G., Wobst, H. J., Kapoor, A., Kenna, L. A. & Southall, N. Clinical development times for innovative drugs. Nat Rev Drug Discov 21, 793 (2022). https://www.nature.com/articles/d41573-021-00190-9
- Sun, D., Gao, W., Hu, H. & Zhou, S. Why 90% of clinical drug development fails and how to improve it? Acta Pharm Sin B 12, 3049 (2022). https://pmc.ncbi.nlm.nih.gov/articles/PMC9293739/
- Mulcahy, A. et al. Use of Clinical Trial Characteristics to Estimate Costs of New Drug Development. JAMA Netw Open 8, e2453275–e2453275 (2025). https://jamanetwork.com/journals/jamanetworkopen/fullarticle/2828689
- Sertkaya, A., Beleche, T., Jessup, A. & Sommers, B. D. Costs of Drug Development and Research and Development Intensity in the US, 2000-2018. JAMA Netw Open 7, e2415445 (2024). https://jamanetwork.com/journals/jamanetworkopen/fullarticle/2820562
- Mullard, A. Parsing clinical success rates. Nat Rev Drug Discov 15, 447 (2016). https://www.nature.com/articles/nrd.2016.136