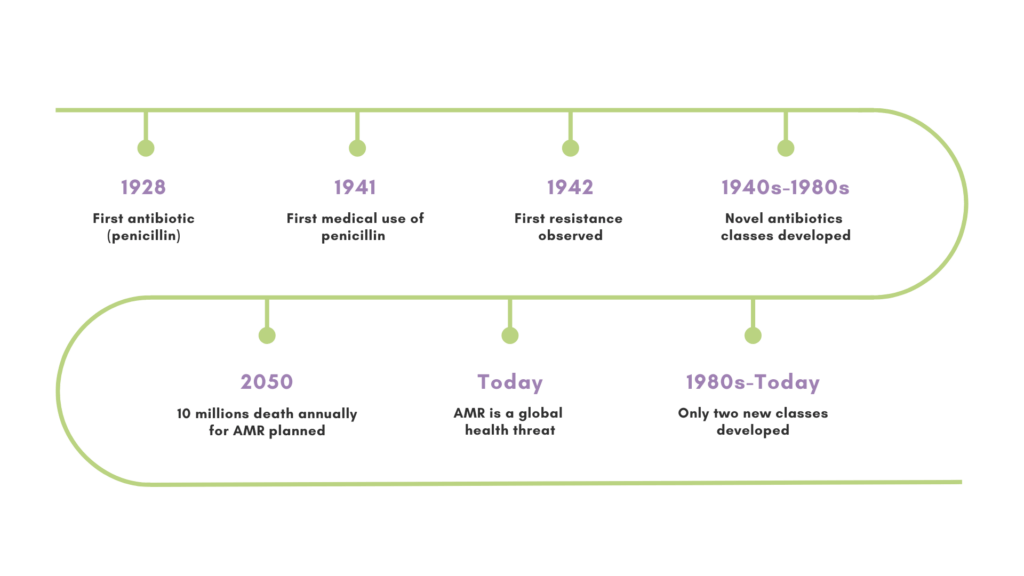
Antimicrobial resistance (AMR) is growing into one of the most significant public health challenges of our time. The World Health Organization claims it is among the main public health threats in the history.
🔬 What is antimicrobial resistance and how did we get here?
AMR happens when microorganisms do become resistant to the drugs that are used to treat the infections they cause. This makes previously treatable diseases more difficult to manage, which therefore leads to longer illness, health complications and an increased risk of death. The story begins with Alexander Fleming’s discovery of penicillin in 1928. Just one year after its first medical use in 1941, the first penicillin-resistant bacterial strain emerged.
🧬 How do bacteria become resistant to antimicrobials?
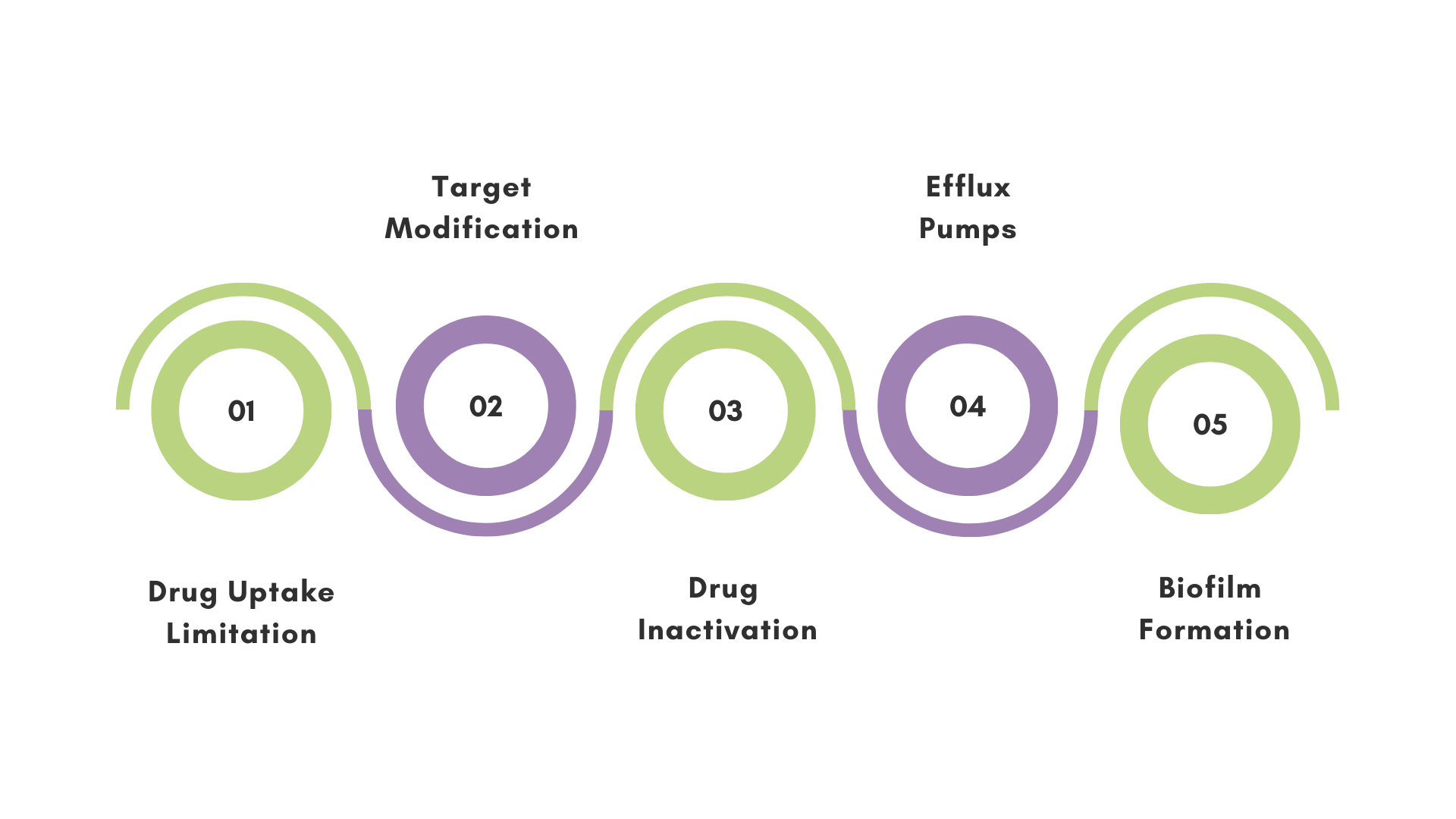
Bacteria can develop resistance through several sophisticated mechanisms such as drug uptake limitation, target modification, drug inactivation, and active efflux using specialized pumps. Over 3900 resistance genes have been classified, those are specific genetic sequences in bacteria that enable them to develop mechanisms of resistance. Bacteria are also able to transfer these genes to each other through different mechanisms, expanding the AMR issue.
🦠 What enhances antimicrobial resistance and its spreading?
Several factors drive the development and spread of AMR:
- Overuse of Antibiotics: Excessive use and misuse of antibiotics, such as prescribing them for viral respiratory infections, contribute significantly to resistance.
- Inadequacy of Treatment: Stopping antibiotic treatment early can allow surviving bacteria to adapt and become resistant.
- Infection Control: Inadequate cleanliness and hygiene in healthcare facilities can promote the spread of resistant infections.
- Agricultural Usage of Antibiotics: In some regions, antibiotics are used in agriculture to promote the growth of healthy animals, leading to the development of resistance that can spread to humans.
🔎 Which approaches can we develop to combat resistance?
Combating AMR requires a multi-faceted approach. Key strategies include judicious use of antibiotics, improved diagnostics and enhanced infection control; but new solutions in research are also emerging, such as AI-driven drug discovery and exploring of natural and synthetic alternative antimicrobial compounds. Following the afore-mentioned strategies, Stop Spread Bad Bugs (SSBB) is a multi-stage consortium with several academic and industrial partners hosting 15 PhD projects, focused on drug discovery and preclinical development of new therapies to address the challenges posed by resistant bacteria.
This blog was written by Alessio Fontanot, one of the PhD candidates working in the SSBB consortium. Alessio is pursuing his PhD trajectory at Erasmus MC University Medical Center Rotterdam in the Netherlands, TissueGnostics and the Medical University of Vienna in Austria.